SIMS Analyses for Beryllium
Secondary ion mass spectrometry
(SIMS, or ion microprobe) represents an extremely sensitive technique for
the microanalysis of beryllium. Positive ions of beryllium are easily formed
during sputtering, with Be showing a useful yield (ions detected/atom sputtered)
of 0.7 (ims 3f) to 1% (ims 6f) with the instrument tuned to maximum transmission.
Such high yields allow sub-ppm quantities to be detected in small volumes
of sample. Just as for other SIMS analyses, one must determine if interfering
species can compromise the analysis. In the case of Be, there is only one
serious interference: triply-charged aluminum (27Al+++). The difference in
mass between 9Be+ and this interference is 0.018 daltons (Be is heavier)
requiring a mass resolving power (M/∆M, 9/0.018) of ~500. This resolving
power can be achieved at very low cost to the signal of interest (maximum
loss of 2x when closing the entrance slit to the mass spectrometer;(Hervig,
2002). Be does not appear to migrate under ion bombardment. In addition,
beryllium is so rare in nature that contamination problems have not been
observed in our lab (however, the analysis of Be-rich phases, such as phenakite
or beryl might lead to a memory effect which could influence subsequent analyses).
A calibration curve for Be in silicate glasses and Be minerals is shown below
(Fig. 1). Matrix effects appear to be small (<20%).
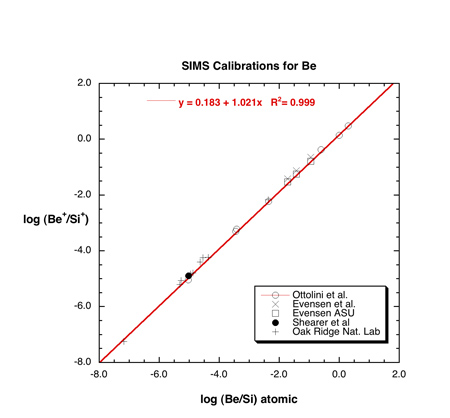
Figure 1: Calibration for Be (redrawn from Hervig, 2002) illustrates the similar
results obtained for Be in four different SIMS laboratories. The analysis conditions
were similar in all cases. The primary beam was O- and positive secondary ions
with a range of excess kinetic energy were analyzed (75 to 100 eV ions with
a bandwidth of ~30-40 eV). Concentrations range from 0.004 ppm Be in San Carlos
olivine (Oak Ridge National Lab analysis) to 164,000 ppm Be in phenakite (Ottolini
et al., 1993). The regression uses only the data set from Ottolini et al. (1993).
Hervig, R.L. (2002) Beryllium analyses by secondary ion mass spectrometry.
In E.S. Grew, Ed. Beryllium: mineralogy, petrology, and geochemistry in the
Earth's crust., 50, p. 319-332. Mineralogical Society of America, Washington,
D. C.
Ottolini, L., Bottazzi, P., and Vannucci, R. (1993) Quantification of lithium,
beryllium, and boron in silicates by secondary ion mass spectrometry using
conventional energy filtering. Anal. Chem., 65, 1960-1968.


