SIMS Analyses for Lithium Concentrations and Isotope Ratios
Secondary ion mass spectrometry (SIMS) is a sensitive technique for the analysis of lithium. Positive ions of this element are easily formed during sputtering with a primary beam of oxygen (lithium has a low ionization potential) and this results in measured useful yields (Li ions detected/Li atoms sputtered) as high as 2 to 4% on the Cameca ims 3f and 6f SIMS at ASU (Hervig et al., 2006). To illustrate the high sensitivity, consider a grain of olivine (Fo90) containing 1 µg/g Li (~3 ppm atomic) Li. This concentration can be represented as ~3 x 1017 atoms Li/cm3 of olivine. If we sputter a cylindrical crater 20 µm in diameter but just 1 µm deep, we will consume ~300 µm3 or 3 x 10-10 cm3 of olivine. Using the atomic density of Li given above, this represents a volume containing ~100 million Li atoms. With a useful yield of 2-4%, we could collect 2-4 million counts of Li during this analysis. This simple calculation is too liberal because the ASU SIMS instruments are single collector mass spectrometers, and would miss counting some 6Li+ while counting 7Li+ (and any other species being monitored). In addition, some signal would be lost because we would partially close the entrance slit to the mass spectrometer to ensure obtaining flat-topped peaks. However, the fact remains that signals for Li will be very high while consuming minimal amounts of a natural sample or experimental run product.
Li calibration
An example of a calibration for lithium is shown in Figure 1. Bulk analyzed rhyolitic and basaltic glasses and two clinopyroxenes are shown. The 7Li+/30Si+ ion ratio was measured using ions with 75±20 eV excess kinetic energy (conventional energy filtering). A least squares regression is shown (forced through the origin). As discussed by Decitre et al. (2002), it does not appear that there is a strong effect of changing sample chemistry on the Li ion yield.
Possible pitfalls with Li analysis by SIMS
Earlier work has documented that Li isotope ratios can also be determined by SIMS on olivine, pyroxene, amphibole, and basaltic glass, and that any matrix effects for these analyses were not detectable (Decitre et al., 2002). However, we are aware of some issues that affect the quality of Li analyses and we describe them below.
Molecular Interferences.
The first item we worry about when attacking an analytical problem by SIMS
is if there are other species at the same nominal mass/charge ratio as
the isotopes of Li. Potential interfering species include 24Mg4+ and 12C2+
on 6Li+, and 28Si4+ and 6LiH+ on 7Li+. A high-resolution mass spectrum
obtained on a water-saturated rhyolitic glass quenched from 500MPa (from
Hervig et al., 2002, AGU abstract) is shown on Figure 2 below. We observe
no sign of either 28Si4+ (simply because it is so much lighter in mass
than 7Li) or 6LiH+ near 7Li+. Similarly, no interfering peaks were observed
near 6Li+. We have not observed the 6LiH+ species in any matrix we have
studied (as of the end of 2006).
Primary Beam Current
While studying
the rhyolitic glass shown in Fig. 2, we noted a dramatic effect of primary
beam current (16O- primary beam accelerated to 12.5kV with an impact energy
at the sample of 17 keV) on the Li+ ion signal. This is shown on Fig. 3, where
the beam current was increased from 0.1 to nearly 2 nA on the hydrous glass
and the natural (~0.2 wt.% H2O) starting material. Note that the Li+/Si+ ratio
decreased by a factor of ~5 as the primary current increased. The starting
material is not strongly affected by the changing beam current, indicating
that the high water content of this sample (>10 wt.%) influences the stability
of the Li ion signal. At the same range of conditions, the Li isotope ratios
were measured on this sample. Increasing the primary current changed the measured
7Li+/6Li+ ratio by ~ 65 ‰ while, again, the nearly dry, glassy starting material
is not strongly affected by changes in primary beam current.
We interpret
the measurements described above to indicate that Li in extremely H2O-rich
rhyolitic glass is mobilized by the primary beam, possibly via a charge-driven
diffusion process. This mobilization affects the light isotope more than the
heavy isotope. This effect is not observed in the starting material containing
low water, and the effect is reduced when the primary beam current is kept
at ≤0.3 nA. Other hydrous samples we have explored do not show such a strong
effect (but have much lower water than the above rhyolite). Lithium-poor, natural
glasses (e.g., Hawaiian basalts) and minerals (e.g., mantle olivine) require
high primary currents to obtain signals intense enough to obtain isotope ratio
measurements. Preliminary tests of olivine do not show any relation between
primary current and measured isotope ratio, but we have observed an effect
on glasses (Fig. 4). As the figure indicates, reproducible analyses of glasses
can be obtained using primary currents greater than a threshold value (about
10 nA in this example). How this threshold varies with current density and
sample composition remains to be explored.
Contamination
Analyses
of some experimental run products indicate a significant gain in Li compared
to the starting material. For example, we have observed a factor of 10 to 100
gain in the Li+/Si+ ion ratio in basalts held in Pt capsules from internally
heated pressure vessels (IHPV) compared to the starting material. We suspect
either that the water added to the capsule was Li-bearing or that the extrusion
process contaminated the tubing at the manufacturing site with a material that
is not removed during acid cleaning of the capsule. Other glassy run products
from experiments using “nano-pure” water and capsules treated with a degreasing
step show low lithium contents similar to their initial concentrations. Vacuum
grease (sometimes used during the preparation of epoxy mounts containing multiple
grains of standards and unknowns) can contain enormous amounts of Li (added
as a "stiffener"), and can result in serious (and even untreatable)
contamination of the sample with lithium. This material should be avoided in
the sample preparation process if the researcher wishes to measure Li in their
sample.
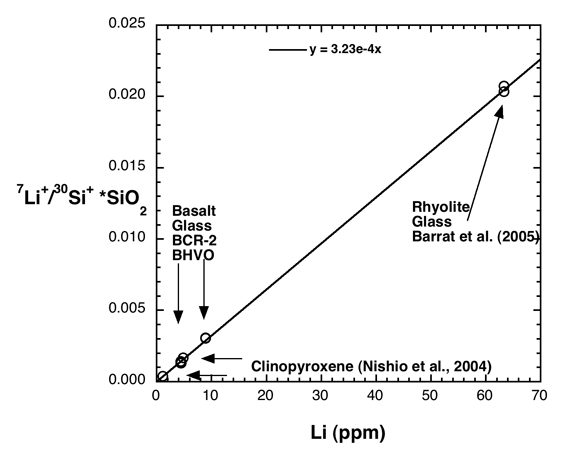
Figure 1. Calibration of the SIMS for lithium. The measured 7Li+/28Si+ ion
ratio is corrected for silica content and compared with bulk analyses of lithium.
The calibration curve intersects clinopyroxenes and basaltic and rhyolitic
glasses, indicating that matrix effects for Li content are small (see also
Decitre et al. (2002).
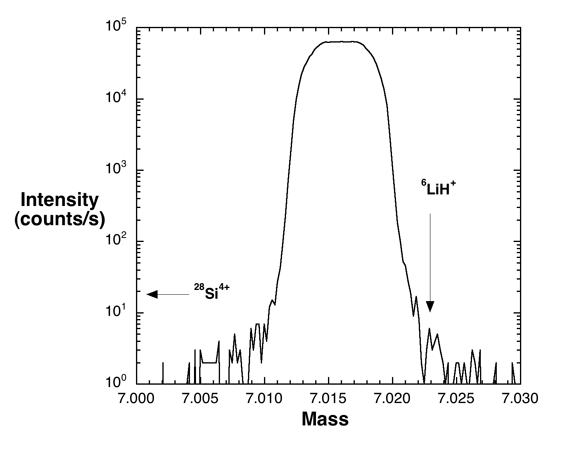
Figure 2. High resolution mass spectrum taken on a hydrated rhyolitic glass.
Despite the high water content (>10 wt.% H2O expected in this sample) there
is no sign of a 6LiH+ molecular ion at the mass/charge predicted (0.007 amu
heavier than 7Li+). A signal from 28Si4+ would be observed 0.02 amu lighter
than 7Li+ (if present). The starting glass contained ~3400 ppmw Li, giving
(at these conditions) about 10 counts/s per ppm Li.
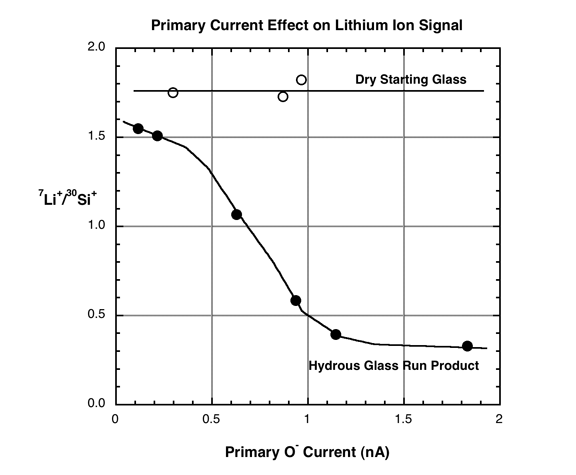 Figure
3. Effect of increasing primary beam current on 7Li+/30Si+ ion signal measured
on macusani rhyolite quenched from 500 MPa (same sample as in Figure 2). The
silicon ion signal increased linearly with the primary current, indicating
that the Li+ signal decreased with increasing primary current.
Figure
3. Effect of increasing primary beam current on 7Li+/30Si+ ion signal measured
on macusani rhyolite quenched from 500 MPa (same sample as in Figure 2). The
silicon ion signal increased linearly with the primary current, indicating
that the Li+ signal decreased with increasing primary current.
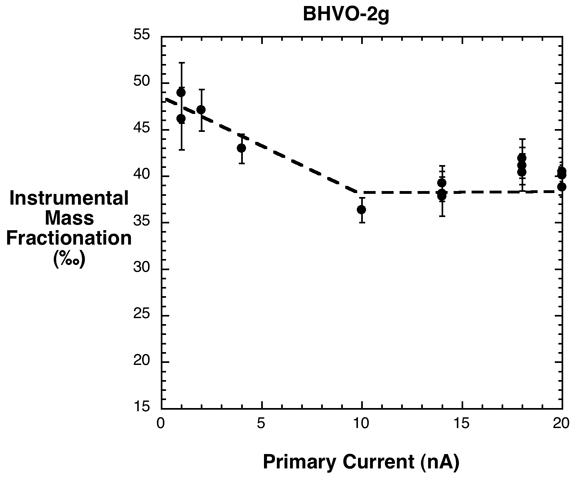
Figure 4. Effect of increasing primary beam current on instrumental mass
fractionation of lithium isotopes measured on "dry" (<0.2 wt.%
H2O) basaltic glass. Instrumental mass fractionation is the deviation (in
‰) of the measured 7Li/6Li ion ratio from the L-SVEC standard ratio (7Li/6Li
= 12.039). Error bars represent 2 standard errors of the mean ratio measured
in each analysis (analyses represent data from two sessions- one on the 3f
and one on the 6f SIMS). The actual Li isotopic composition of this basalt
is ~+4‰.
Decitre, S., Deloule, E., Reisberg, L., James, R.H., Agrinier, P., and Mevel,
C. (2002) Behavior of Li and its isotopes during serpentinization of oceanic
peridotites. Geochemistry, Geophysics, Geosystems, 3(1), 10.1029/2001GC000178.
Hervig, R.L., Mazdab, F.K., Williams, P., Guan, Y., Huss, G.R., and Leshin,
L.A. (2006) Useful ion yields for Cameca IMS 3f and 6f SIMS: Limits on quantitative
analysis. Chemical Geology, 227, 83-99.


