Self diffusion of network formers (silicon and oxygen) in naturally occurring basaltic liquid
Geochemica et Cosmochimica Acta, Vol. 60, No. 3, pp. 405-413,
1996
Copyright © 1995 Elsevier Science Ltd.
C. E. Lesher1, R. L. Hervig2, and D. Tinker1
1Department of Geology, University of California, Davis, CA
95616, USA
2School of Earth and Space Exploration,
Arizona State University, Tempe, AZ 85287, USA
(Received October 14, 1994; accepted in revised form October 26, 1995)
Abstract
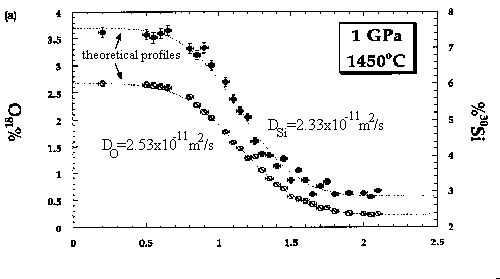
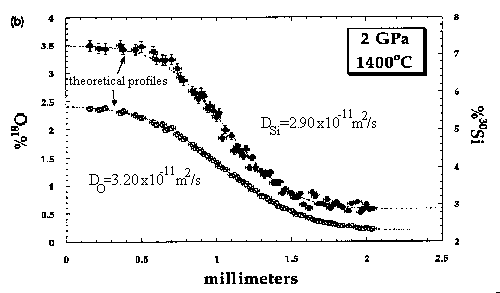
Self diffusion coefficients (D) for silicon and oxygen in anhydrous basaltic liquid [O/(Si + Al) = 2.5] were measured at 1 and 2 GPa and temperatures between 1320 and 1600°C. Simple diffusion couples were composed of isotopically normal basaltic glass synthesized from chemical reagents mated to chemically identical glass enriched in 18O and 30Si. Concentrations of 18O and 30Si across the interfacial region of the couples were analyzed by ion microprobe. At 1 and 2 GPa, DO is consistently larger than DSi for a given diffusion couple, but only at the highest temperature (1600°C) is the difference outside the small uncertainties for the analytical measurements. At 1 GPa the self diffusivities for both Si and O are well-described by the Arrhenius relationship
where T is temperature in K, R is the gas constant in J K-1 mole-1, and D is expressed in m2s-1. Self diffusion coefficients at 2 GPa, are a factor of 1.5 greater and at 1400°C the activation volume (Va) is -6.7 cm3 mol-1. The similarity in self diffusion coefficients, small activation energies (<50% of the Si-O bonding energy), and negative activation volumes for Si and O self-diffusion in basaltic liquid suggest that network former diffusion is a largely cooperative process involving local contraction of the anionic structure. An evaluation of the Eyring n-D relationship implies a mean translation distance for network former diffusion that is 2 - 3 times the diameter of the oxygen ion and of the order of the Si-Si separation distance. These features of network former diffusion are consistent with the formation of high-coordinated Si as a transition complex in melts populated by Q2, Q3, and Q4 species. In light of inferred changes in melt structure with increasing silica content, we further speculate that the dominant mode of network former diffusion changes from a cooperative process in basaltic liquid, perhaps involving SiO5 transition complexes, to an ionic process (Si4+ and O2-) in liquids approaching full polymerization.